Soap is a familiar household item, yet its function is often taken for granted. Beyond its role in hygiene, soap represents a fascinating intersection of chemistry, biology, and everyday life. Understanding how soap works not only enhances our appreciation of this commonplace substance but also informs better practices in cleaning, public health, and personal care. From removing grease in the kitchen to reducing the spread of infectious diseases, the molecular mechanics of soap are both elegant and highly effective.
The Molecular Structure of Soap
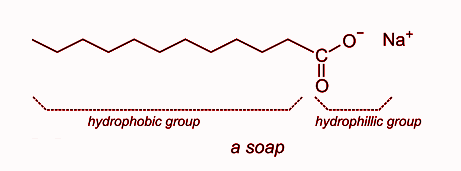
At its core, soap is a molecule designed to bridge two worlds: water and oil. Chemically, a typical soap molecule has a dual nature:
- Hydrophilic (water-loving) head: This part of the molecule readily bonds with water molecules, enabling it to dissolve in aqueous environments.
- Hydrophobic (water-fearing) tail: Composed of a long chain of hydrocarbons, this tail avoids water and instead binds to nonpolar substances like oils, fats, and grease.
This amphiphilic structure—having both hydrophilic and hydrophobic regions—is what gives soap its remarkable cleaning ability. It allows soap molecules to surround oily dirt particles, forming structures called micelles, which trap the grease in the center while the hydrophilic heads interact with the surrounding water. As a result, dirt that would otherwise resist water alone can be suspended and rinsed away.
How Soap Removes Dirt
Soap’s action is a combination of physical and chemical processes:
Breaking Down Oils and Fats
Many forms of dirt, from cooking grease to skin oils, are hydrophobic. Water alone cannot dissolve these substances. When soap is introduced, the hydrophobic tails attach to the oil particles while the hydrophilic heads face outward toward the water. As soap molecules cluster around the oil, they form micelles that isolate and lift grease from surfaces. Agitation—like scrubbing hands or washing dishes—helps disperse these micelles, allowing the encapsulated oil to be rinsed away.
Reducing Surface Tension
Water naturally forms a high surface tension, making it less effective at penetrating greasy or sticky surfaces. Soap molecules disrupt this tension by positioning themselves at the water-air interface, reducing cohesive forces. This allows water to spread more easily and wet surfaces thoroughly, enhancing its ability to lift dirt.
Removing Particulate Matter
Besides oils, soap also helps suspend particulate matter such as dust, soil, or small debris. Micelles can surround both oily and solid particles, effectively emulsifying them in water. This combination of chemical binding and mechanical removal is why washing with soap is vastly more effective than rinsing with water alone.
The Antimicrobial Effects of Soap
One of soap’s most celebrated benefits is its ability to combat germs. While many cleaning agents kill bacteria or viruses chemically, soap primarily neutralizes microbes through physical disruption.
Disrupting Lipid Membranes
Many pathogens, including enveloped viruses like influenza or SARS-CoV-2, are encased in lipid membranes. The hydrophobic tails of soap molecules embed into these lipid layers, destabilizing them. As the membrane disintegrates, the virus or bacterium loses structural integrity and becomes inactive.
Washing Away Microbes
Even microbes not directly destroyed by soap are removed through the formation of micelles. Pathogens adhere to the oil and dirt on skin or surfaces, and as soap encapsulates these particles, they are carried away with water during rinsing. This physical removal is particularly important in everyday hand hygiene, significantly reducing the risk of infection.
The Science Behind Lather and Foam
Many people equate soap with bubbles and froth, but the foam itself is not responsible for cleaning. Foam results from air being trapped in a thin layer of soap and water. It indicates the presence of surfactants, but the effectiveness of soap depends on micelle formation rather than lather volume.
Interestingly, certain soaps and detergents produce more foam than others because of additives or molecular structure, but a low-foam soap can clean just as effectively. The real measure of soap’s cleaning power lies in its ability to emulsify oils and suspend debris, not the amount of foam it generates.
The History and Cultural Significance of Soap
Soap has been part of human civilization for millennia. Archaeological evidence suggests that as early as 2800 BCE, people in Babylon were using mixtures of water, alkali, and animal fats to clean. Roman texts reference soap-like substances used for both bathing and laundry, while medieval Europe saw the rise of soapmaking as a specialized craft.
Culturally, soap has been tied to notions of cleanliness, morality, and social status. Public bathhouses in ancient societies were not just hygienic spaces—they were centers for social interaction and personal care. In modern times, soap remains a symbol of health, from personal hygiene routines to public health campaigns, especially in preventing disease outbreaks.
Soap Variations: From Traditional to Modern

Soap is not a single substance; it encompasses a range of products formulated for different purposes:
- Bar soap: Traditional solid form, often made from saponified animal fats or vegetable oils. Effective, economical, and long-lasting.
- Liquid soap: Contains similar surfactants as bar soap but often includes additional moisturizers or antimicrobial agents.
- Antibacterial soap: Contains chemicals like triclosan, aimed at killing bacteria. However, studies show that regular soap and proper handwashing are often just as effective.
- Specialty soaps: Formulated for sensitive skin, exfoliation, or fragrance purposes. Ingredients may vary widely, but the underlying cleaning mechanism remains the same.
Understanding these variations helps consumers choose products based on skin type, usage context, and environmental considerations.
Environmental and Health Considerations
While soap is generally safe, certain ingredients can have ecological or health impacts:
- Phosphates and sulfates: Can contribute to water pollution, harming aquatic life.
- Synthetic fragrances and preservatives: Occasionally cause allergic reactions or skin irritation in sensitive individuals.
- Biodegradability: Traditional soaps made from natural fats and alkalis tend to break down more readily than some synthetic detergents.
Choosing biodegradable soaps with minimal harmful additives supports both personal health and environmental sustainability. Additionally, proper use—avoiding excessive quantities—reduces waste and environmental burden.
Practical Tips for Effective Soap Use
Even the most chemically advanced soap will underperform without proper technique. Key practices include:
- Duration: Handwashing should last at least 20 seconds to allow micelles to form and pathogens to be removed.
- Coverage: Ensure all skin surfaces, including between fingers and under nails, are cleaned thoroughly.
- Temperature: Warm water helps dissolve oils, but cold water with soap is still effective for mechanical removal.
- Rinsing: Proper rinsing ensures that emulsified dirt, oils, and microbes are washed away rather than left on the skin.
These simple practices magnify soap’s chemical efficacy, making everyday cleaning significantly more effective.
Key Takeaways
- Soap molecules are amphiphilic, with hydrophilic heads and hydrophobic tails, allowing them to emulsify oils and suspend dirt.
- Soap reduces water’s surface tension, improving its ability to penetrate and clean surfaces.
- Micelles are the structures that trap oils, dirt, and microbes for easy removal.
- Soap physically disrupts lipid membranes of many pathogens, inactivating viruses and bacteria.
- Foam indicates the presence of surfactants but does not directly correlate with cleaning efficiency.
- Historical soap use spans millennia, reflecting both hygiene and cultural values.
- Different soap formulations exist, but the basic cleaning mechanism remains the same.
- Proper usage—duration, coverage, and rinsing—is essential to maximize effectiveness.
Frequently Asked Questions (FAQ)
Q1: Can soap kill all bacteria and viruses?
A1: Soap is effective against many pathogens, particularly those with lipid membranes, but it may not inactivate all bacteria or viruses chemically. Physical removal through washing is equally important.
Q2: Is antibacterial soap better than regular soap?
A2: Studies show that regular soap is generally as effective as antibacterial formulations for everyday handwashing, without the risk of promoting chemical resistance.
Q3: Does more foam mean better cleaning?
A3: No. Foam is a visual indicator of surfactants but does not measure cleaning power. Micelle formation is the key factor.
Q4: Can soap remove heavy grease from kitchen surfaces?
A4: Yes. Soap’s hydrophobic tails bind to grease, forming micelles that can be rinsed away with water. Agitation improves effectiveness.
Q5: Are all soaps environmentally friendly?
A5: Not all. Natural, biodegradable soaps break down more easily and have fewer harmful additives than synthetic detergents with phosphates or certain preservatives.
Conclusion
Soap is a simple yet sophisticated tool, leveraging fundamental chemical principles to address everyday challenges of hygiene and cleanliness. Its amphiphilic molecules can trap oils, dirt, and microbes, making them easily removable with water. Beyond the laboratory, soap has a rich historical and cultural significance, influencing social norms and public health practices. By understanding how soap works and using it effectively, we harness the power of chemistry in our daily lives, protecting ourselves and our communities while maintaining a cleaner, healthier environment.
