Atmospheric chemistry is the scientific study of the chemical composition of the Earth’s atmosphere and the reactions and interactions that occur among its various components. This field is central to understanding climate change, air pollution, and environmental health. By examining the molecular-level processes that govern the behavior of gases, aerosols, and reactive species, scientists can predict and mitigate the effects of human activity on the planet.
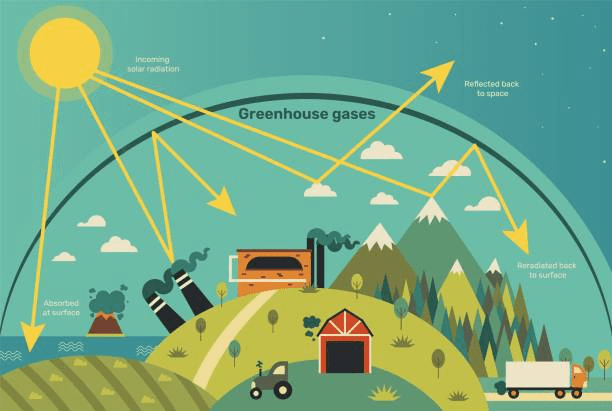
The complexity of atmospheric chemistry arises from the interplay between natural processes, such as volcanic eruptions and plant emissions, and anthropogenic influences, including fossil fuel combustion, industrial emissions, and deforestation. These factors contribute to the concentration of greenhouse gases, the formation of ozone, and the presence of particulate matter, all of which have profound effects on climate, ecosystems, and human health.
This article explores the core principles of atmospheric chemistry, its role in climate change, the molecular reactions that drive these phenomena, and the techniques used to monitor and model atmospheric processes.
The Composition of Earth’s Atmosphere
The Earth’s atmosphere is a dynamic mixture of gases, liquids, and solids, interacting through complex chemical and physical processes. While nitrogen (N₂) and oxygen (O₂) dominate the atmosphere, trace gases such as carbon dioxide (CO₂), methane (CH₄), ozone (O₃), and nitrous oxide (N₂O) play disproportionate roles in climate regulation and chemical reactivity.
Greenhouse Gases and Climate Impact
Greenhouse gases are critical components of atmospheric chemistry because of their ability to trap infrared radiation, contributing to the greenhouse effect and global warming. Carbon dioxide, methane, and nitrous oxide, despite their relatively low concentrations, significantly influence the Earth’s energy balance.
The molecular interactions of these gases with radiation involve the absorption and emission of specific wavelengths of energy, which heats the lower atmosphere and surface. Methane, for instance, is over 25 times more effective than CO₂ at trapping heat over a 100-year period, highlighting the importance of monitoring even trace amounts of reactive gases.
Reactive Atmospheric Species
In addition to stable greenhouse gases, the atmosphere contains highly reactive species such as hydroxyl radicals (OH), ozone, and nitrogen oxides (NOₓ). These species drive chemical reactions that regulate the concentration of greenhouse gases and pollutants. For example, hydroxyl radicals act as the “detergent” of the atmosphere, initiating the breakdown of methane and other volatile organic compounds (VOCs), thereby influencing atmospheric lifetimes and climate feedback loops.
Chemical Reactions Driving Climate Change
Atmospheric chemistry is governed by a multitude of chemical reactions, from simple photolysis to complex chain reactions. Understanding these reactions at the molecular level is essential to predicting climate change and assessing mitigation strategies.
Photochemical Reactions
Sunlight drives many critical reactions in the atmosphere. Photolysis, the breaking of chemical bonds by photons, initiates processes that produce ozone in the stratosphere and troposphere. For example, the photolysis of molecular oxygen (O₂) produces atomic oxygen, which then reacts with O₂ to form ozone (O₃).
Stratospheric ozone protects life on Earth by absorbing ultraviolet (UV) radiation, whereas tropospheric ozone contributes to smog formation and respiratory problems. The balance of ozone production and destruction is sensitive to human emissions of nitrogen oxides and chlorofluorocarbons (CFCs), demonstrating the intricate link between molecular chemistry and climate.
Greenhouse Gas Reactions
Greenhouse gases undergo various reactions that alter their concentration and impact. Methane, emitted from natural and anthropogenic sources, reacts with hydroxyl radicals in the troposphere, forming water vapor and carbon dioxide. These reactions influence not only climate warming but also the oxidative capacity of the atmosphere, affecting the removal of other pollutants.
Nitrous oxide, a potent greenhouse gas, participates in photochemical reactions that produce reactive nitrogen species. These species contribute to ozone depletion in the stratosphere and the formation of nitric acid in the troposphere, linking climate change to air quality and environmental chemistry.
Aerosol Formation and Chemistry
Aerosols, tiny particles suspended in the air, play a dual role in climate by scattering and absorbing sunlight and acting as cloud condensation nuclei. Atmospheric chemistry governs the formation, growth, and transformation of aerosols through reactions involving sulfur dioxide (SO₂), nitrogen oxides, ammonia, and organic compounds. These reactions can lead to acid rain, reduced visibility, and changes in cloud reflectivity, impacting both local weather and global climate patterns.
| Atmospheric Component | Key Chemical Processes | Impact on Climate |
|---|---|---|
| CO₂ | Absorption and emission of infrared radiation | Global warming |
| CH₄ | Oxidation by hydroxyl radicals | Greenhouse effect amplification |
| O₃ | Photolysis and radical reactions | UV protection and smog formation |
| Aerosols | Sulfate and organic reactions | Cloud formation, albedo effect |
| N₂O | Photochemical oxidation | Ozone depletion and greenhouse effect |
This table illustrates the interconnection between molecular processes and broader climatic impacts.
Monitoring and Modeling Atmospheric Chemistry
To understand and predict the consequences of human activity on climate, scientists rely on advanced monitoring and modeling techniques. These approaches quantify the concentration, distribution, and reactivity of atmospheric components.
Remote Sensing and In-Situ Measurement
Satellites equipped with spectrometers detect trace gases and aerosols globally, measuring concentrations of CO₂, CH₄, ozone, and other key compounds. Ground-based instruments, such as gas analyzers and lidar systems, complement satellite data by providing high-resolution, localized measurements. Together, these tools enable comprehensive monitoring of atmospheric chemistry in near-real-time.
Computational Modeling
Atmospheric chemists use complex computational models to simulate chemical reactions, transport, and interactions within the atmosphere. These models integrate data from laboratory studies, field measurements, and satellite observations. By simulating scenarios of greenhouse gas emissions, aerosol loading, and chemical transformations, scientists can project climate trends, assess mitigation strategies, and guide policy decisions.
Role in Climate Policy
Accurate knowledge of atmospheric chemistry is essential for effective climate policy. Understanding molecular-level processes informs strategies for reducing greenhouse gas emissions, controlling pollutants, and managing air quality. International agreements, such as the Paris Agreement, rely on scientific evidence derived from atmospheric chemistry research to set emission targets and monitor progress.
Implications for Environmental Health and Sustainability
The study of atmospheric chemistry extends beyond climate modeling to public health, ecosystem stability, and sustainability initiatives.
Air Quality and Human Health
Reactive gases and aerosols directly affect respiratory and cardiovascular health. Tropospheric ozone, particulate matter, and nitrogen oxides contribute to asthma, lung disease, and premature mortality. Understanding the chemical pathways that produce these pollutants is critical for designing effective mitigation strategies and regulatory policies.
Ecosystem Impacts
Atmospheric chemical processes influence ecosystems through acid deposition, nutrient cycling, and UV radiation exposure. Acid rain, formed from reactions of sulfur dioxide and nitrogen oxides, damages forests, soils, and aquatic systems. Elevated ozone and UV radiation affect plant growth and crop productivity, linking molecular atmospheric chemistry to food security and biodiversity.
Sustainability and Mitigation Strategies
Reducing the environmental impact of human activity requires a molecular-level understanding of atmospheric chemistry. Strategies include transitioning to low-carbon energy sources, controlling industrial emissions, enhancing urban air quality, and implementing carbon capture technologies. By targeting the chemical processes that drive climate change, these approaches aim to stabilize the atmosphere and mitigate long-term risks.
Conclusion
Atmospheric chemistry provides a critical framework for understanding climate change at the molecular level. By examining the behavior and interactions of greenhouse gases, reactive species, and aerosols, scientists can elucidate the mechanisms driving global warming, air pollution, and ecological disruption.
From the photochemical reactions that generate ozone to the oxidation of methane and the formation of aerosols, molecular processes govern both the chemistry and physics of our atmosphere. Monitoring, modeling, and intervention strategies are essential to mitigate the effects of human activity, protect public health, and promote environmental sustainability.
Ultimately, the study of atmospheric chemistry bridges the gap between microscopic molecular phenomena and global environmental challenges. It highlights the necessity of integrating science, technology, and policy to address climate change, ensuring a healthier, more resilient planet for future generations.
