Physics

The Physics of Wireless Charging: How Electromagnetic Fields Power Your Devices
user243
Dropping a phone onto a pad and watching the battery icon light up feels like magic, yet the process is ...

The Mole and Avogadro’s Constant
Charlie Waters
If you were searching for methods to get rid of the moles that are ruining your garden, then unfortunately this ...
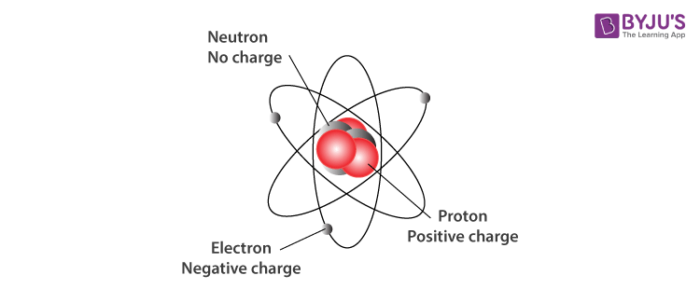
Subatomic Particles In An Atom: Their Discovery and Properties
admin
Before talking about subatomic particles, let’s know what an atom is. Atoms are the building blocks of matters. It is ...
Atomic Structure And Isotopes And Isotopic Notation
Charlie Waters
The smallest units that make up all matter are called atoms. Atoms of different elements have unique properties and behave ...






