Acids have always been a substance of fear, something we try to stay away from. While there are harmless weak acids, there are also very strong acids that are harmful to even touch. But there is another type of acid we don’t hear about much. Even inside a chemistry lab, they are subjects of extreme caution. They are the mysterious superacids. And they are so strong that people question their existence. Today we will try to discuss this idea of superacid and a few of its members.
What is Acid?
You probably already know what an acid is. In a normal sense, acids are sour and astringent substances. A very common example would be lemon juice which contains citric acids or the must-have vinegar which is just watered-down acetic acid. Very familiar right?
In chemistry, an acid is a molecule or ion that can accept electron pairs or donate protons (hydrogen ion, H+). For example, acetic acid donates a proton to baking soda (NaHCO3) in the following manner
CH3COOH + NaHCO3 = CH3COONa + CO2 + H2O
You can see that an H+ was donated and Na+ took its position. By the way, this is a fun little experiment that you can try out at your home. It produces a lot of CO2 which comes out in the form of bubbles. The reaction is vigorous but safe. Be sure to use them in small amounts or someone is going to get scolded (or beaten) by their m😡m.
Strength of Acid or Acidity
The examples given earlier do not sound so scary. So, what about the warnings we hear so much? The warnings are indeed correct. Acids can be divided into two types:
- Weak organic acids e.g., ethanoic acid (vinegar)
2. Strong mineral acids such as hydrochloric acid (HCl), nitric acid (HNO3), etc.
While superacid is an acid that is stronger than even pure sulfuric acid. We will come to it later. Superacid is best served cold.
Let’s talk about the strength of acids first. As said earlier, organic acids are weaker while mineral acids are strong. But what is this strength based on and how can you measure it?
The strength of an acid solution is often expressed in terms of the number of hydronium ions (H3O+) it can generate in a water solution.
HCl + H2O = H3O+ + Cl–
The amount of H3O+ is measured in the concentration unit molL-1 or shorter “M” (pronounced as molar). The more the concentration of H3O+, the stronger is the acid.
The pH Scale
But there is a better way of comparing the strength. That is by calculating the negative logarithmic value of concentration.
This value is called pH value and was introduced by Danish chemist Søren Sørensen in 1909.
So, pH= -log[H3O+]; here [H3O+] = concentration of hydronium ion
The negative sign means that the pH and strength are opposite to each other. The stronger the acid the lower the value of the pH is. And for each unit of pH that decreases, the strength of the acid increases by 10 times!!
That is not all. The pH can have a value ranging from 0 to 14 which is called the pH scale. The pH value 7 means a neutral solution. As the value increases the solution becomes more alkaline. And you already know that as the pH decreases the solution becomes more acidic.
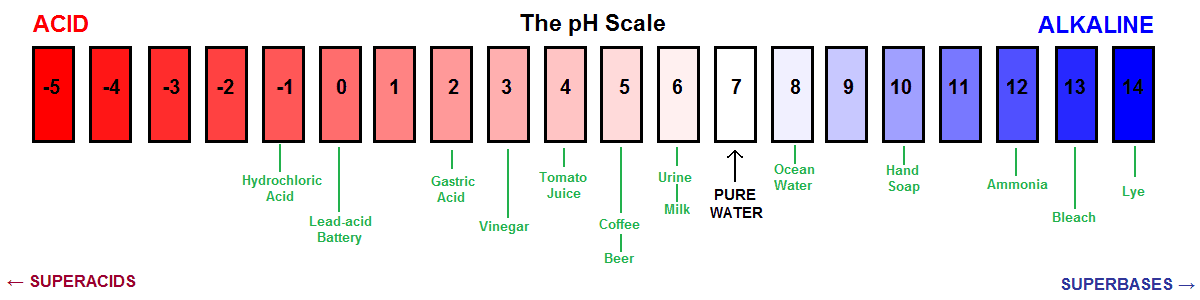
But as a matter of fact, all acids that have a pH value between 0 and 7 are considered weak inside a chemistry lab. Wait a moment. Stomach acid contains HCl and it has a pH between 1 and 2. So, shouldn’t it be a weak acid? No, HCl is a strong acid but it can have a dilute solution. You can make solutions of varying concentrations.
If you make a 1 M solution of HCl it produces the same amount of H3O+ ions and the pH will be 0. The pH of 1 M acetic acid should also be 0 then, right? Bzzzzzz. Wrong answer. The pH value of 1 M acetic acid is 2.38 meaning it is about 240 times weaker than the 1 M HCl solution!! This is because acetic acid is a weak acid and doesn’t produce as many H3O+ ions and thus weaker. What does it produce less? That’s another big explanation. Let’s forget that question for now.
Strong acids vs. The pH Scale
A question should be popping up in your mind by now, “How much concentrated acid solutions can we make?” You will be surprised to know that it is possible to make a 38% HCl solution that has a concentration of almost 12 M!! For sulfuric acid that would be 18.4 M!! Any more than that is out of water capacity. And that’s what you call strong.
We are almost in the zone of superacid, just a bit more.
So, what would be the pH of 12 M HCl? It should be -12. Although it is mathematically correct, it is not chemically possible. Few reasons being
- At high concentrations, even strong acids do not dissociate fully in water and produce a lower number of H3O+ ions.
- The amount of water per acid unit simply becomes inefficient.
- Moreover, the pH meters don’t work at high acidity due to “acid error”.
- One more important fact is that the acid species being produced changes which greatly changes the activity coefficient of the ions.
These are but a few reasons. Also, a lot of chemistry goes into explaining them.
There is yet another problem that is the “leveling effect”. You have to go back a bit to understand this. The pH of 1 M HCl is 0. And it is the same for 1 M HNO3 because it too is a strong acid. The same goes for other strong acids too. Did you see what just happened? All of them got leveled to the same strength. This is because whatever acid you put into water; the water turns it into an H3O+ ion. How envious is he!! As water is the solvent, it is his rule.
Because of all these reasons, pH is used only in measuring weak acid solutions.
As a result, when comparing superacids, we cannot use pH or anything that involves mixing acids into water.
The Hammett acidity function
So, what do we do now? This was answered by physical organic chemist Louis Plack Hammett.
He proposed the use of an acidity function that omits the water part from the pH equation and can be used for concentrated solutions of strong acids. The acidity function is called the Hammett acidity function and is denoted by H0.
The Hammett acidity function,
H0 = pKBH+ + log BBH+
H0 is analogous to pH and it is as if the pH scale has been extended to the negative direction.
Moreover, the value of H0 is equivalent to pH assuming the acid dissociated normally in water. But that is not the case. So, remember that H0= -12 does not mean that the pH is also -12.
Superacid
After listening to all those things, we can finally talk about the superacid. It’s obvious from the name that superacids are acids that are so strong that they cannot only be called strong acids anymore.
So how strong will an acid have to be before you can call it a superacid? Well, pure sulfuric acid is the strongest acid that you can find in nature. As said earlier, it has an of -12.
Any acid that is stronger than 100% pure sulfuric acid i.e., that has an H0 value lower than -12 is called a superacid. Superacids are generally a medium where the chemical potential of a proton is higher than in pure sulfuric acid.
There are a lot of compounds that do not react with even sulfuric acid but very few compounds can ignore superacids.
The idea of superacid was known from as early as 1927. But it became popular when George A. Olah first prepared a superacid called the magic acid, a 1:1 mixture of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). He was working with hydrocarbons and found that a superacid can produce carbonium ion or carbocation, an ion with a positively charged carbon in it. Carbonium ions are very important in polymer making. He won the Nobel prize in 1994 for his works in carbocations.
If you are wondering about the name, magic acid, it came up when coined after chemists placed a paraffin candle in a sample of magic acid after a Christmas party. The candle was dissolved almost instantly, showing the ability of the acid to attack hydrocarbons. Please, don’t even think of using a superacid for a magic trick, you know why.
Let’s look at a few examples of superacids and how dangerous they are. After all, that is what we all are after, right? Haha. So, without further ado, let’s start.
Triflic Acid
Triflic acid is a superacid with an H0 value of -14.9. This means that it is 1 a thousand times stronger than pure sulfuric acid. The full name of triflic acid is trifluoromethanesulfonic acid and it has the chemical formula CF3SO3H.
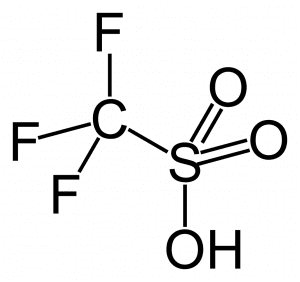
It has strong protonating power and is used as a catalyst in many organic reactions where hydrochloric acid or sulfuric acid are only moderately strong and won’t serve the purpose.
If it touches the skin it will give immediate burn to the skin and even damage tissues underneath. On inhalation, it causes fatal spasms.
Magic Acid
As mentioned earlier, magic acid is a 1:1 mixture of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). It is prepared by mixing hydrofluoric acid (HF), sulfur trioxide (SO3) antimony pentafluoride (SbF5). It has an H0 value starting from -19.2 up to 23 depending on the ratio of mixture components. Meaning it is 100 million times stronger than triflic acid and 100 billion times stronger than pure sulfuric acid!!
When comes in contact, it will cause serious injury if not death. It is so reactive that is kept under nitrogen which creates an inert surrounding.
It is used to produce higher-quality gasoline from crude fuels. The carbocation produces forms more complex bonds between them resulting in higher grade fuel.
SbF5 + HSO3F + CH4 = F5Sb − OSO2F−+CH+5
Still, it is not the strongest acid on earth. That position goes to Fluoroantimonic acid.
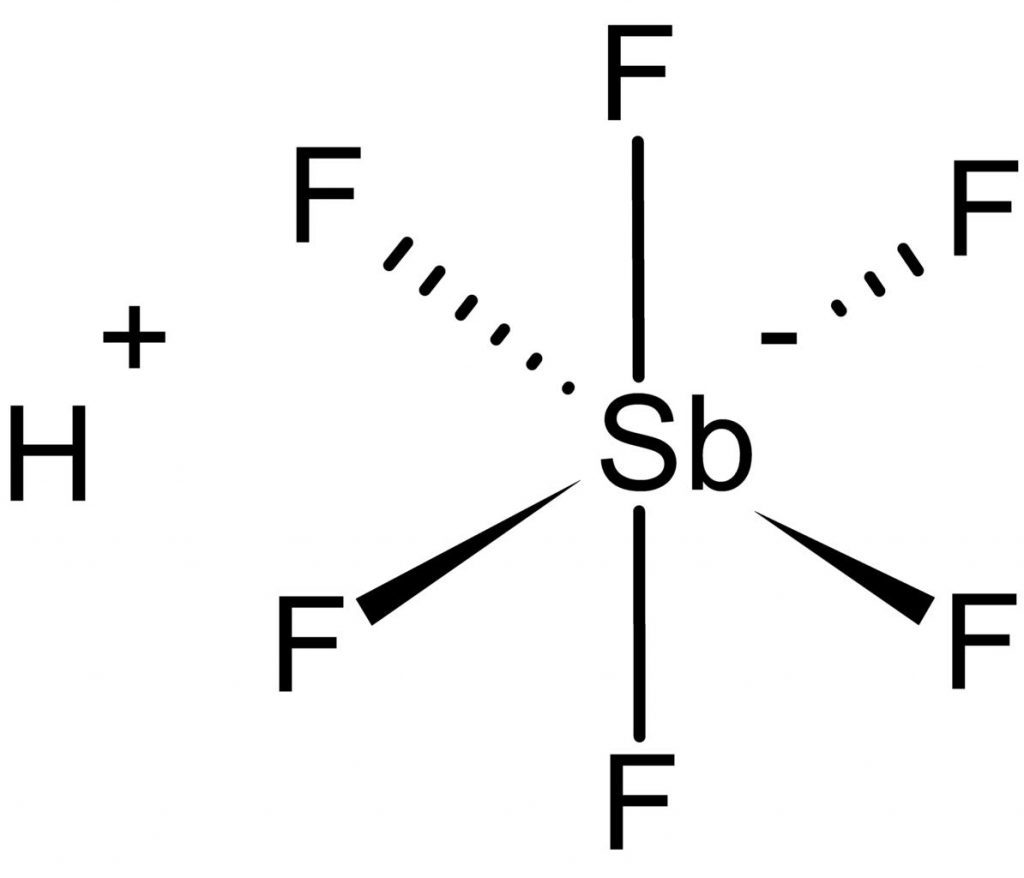
Fluoroantimonic Acid- World’s Strongest Acid
This is the strongest and most reactive acid known to man to this day. It is prepared by combining hydrogen fluoride (HF) with antimony pentafluoride (SbF5). The resulting acid is a monster with an H0 value of -28. That’s 10,000 times stronger than even the magic acid!! And 10 quadrillion times or 10 million billion times stronger than pure sulfuric acid!!!
It will not just burn you or not just kill you. It will eat through skin flesh bones everything. No matter what container you put it in, it will dissolve that and keep marching ahead except Teflon.
Yes, it only bows before Teflon. And used to produce the container for Fluoroantimonic acid. The very material used to make your frying pan. No need to thank me for giving you this good news.
So why Teflon? Let’s look at the acid first. You will notice that almost all superacids contain the element fluorine. When fluorine-containing acids are mixed, the fluorine gets attached to multiple hydrogen ions. And it will give up the hydrogen ions readily for just about anything.
But Teflon is a polymer of tetrafluoroethylene molecules that already contains strong carbon-fluorine bonds and will not lose to even the strongest of acids.
That’s it for today. If you ever come across any super acid, it is probably best to stay away from it.
For now, we have frying pans, sorry, Teflon. If humans will ever produce an acid that can’t be stopped by anything is not a certainty. What certain is that we should always focus on what’s good for humanity, not just superacids but everything around us.
If you like the content, visit Chemniverse.com for more like this.
Have a nice day!!